Nature Nanotechnology
Volume 19
pages 705–714 (2024)
Author: Jack P. M. Andrews, Shruti S. Joshi, Evangelos Tzolos, Maaz B. Syed, Hayley Cuthbert, Livia E. Crica, Neus Lozano, Emmanuel Okwelogu, Jennifer B. Raftis, Lorraine Bruce, Craig A. Poland, Rodger Duffin, Paul H. B. Fokkens, A. John F. Boere, Daan L. A. C. Leseman, Ian L. Megson, Phil D. Whitfield, Kerstin Ziegler, Seshu Tammireddy, Marilena Hadjidemetriou, Cyrill Bussy, Flemming R. Cassee, David E. Newby, Kostas Kostarelos & Mark R. Miller
Abstract
Graphene oxide nanomaterials are being developed for wide-ranging applications but are associated with potential safety concerns for human health. We conducted a double-blind randomized controlled study to determine how the inhalation of graphene oxide nanosheets affects acute pulmonary and cardiovascular function. Small and ultrasmall graphene oxide nanosheets at a concentration of 200 μg m−3 or filtered air were inhaled for 2 h by 14 young healthy volunteers in repeated visits. Overall, graphene oxide nanosheet exposure was well tolerated with no adverse effects. Heart rate, blood pressure, lung function and inflammatory markers were unaffected irrespective of graphene oxide particle size. Highly enriched blood proteomics analysis revealed very few differential plasma proteins and thrombus formation was mildly increased in an ex vivo model of arterial injury. Overall, acute inhalation of highly purified and thin nanometre-sized graphene oxide nanosheets was not associated with overt detrimental effects in healthy humans. These findings demonstrate the feasibility of carefully controlled human exposures at a clinical setting for risk assessment of graphene oxide, and lay the foundations for investigating the effects of other two-dimensional nanomaterials in humans. Clinicaltrials.gov ref: NCT03659864.
Main
Two-dimensional (2D) nanomaterials are defined as flat, non-spherically shaped substances with a least one dimension <100 nm, and have generated worldwide interest for a variety of potential applications including building materials, car tyres, inks, food preservatives, sun-screens and anti-corrosion and lubricating products. Graphene is an archetypal 2D nanomaterial of a single layer or few layers of carbon lattice. Its unique structure, strength, flexibility, transparency and electrical conductance properties make it attractive for a wide range of applications. There is also intense interest in further developing such materials for biomedical applications, including diagnostic and drug-delivery agents. The oxidized form of graphene, graphene oxide (GO), has shown promise in the biomedical setting due to its hydrophilicity, high surface area for chemical functionalization, reasonable colloidal stability in biologically relevant solutions and compatibility with blood cells. However, like other manufactured nanomaterials, the safety profile and limitations of GO on human exposure need to be determined before widespread use. There are limited and inconsistent toxicological data available for GO, often arising from differences among the many different sources of the material and their notable variability in dimensions and chemical properties that do not allow confident conclusions to be reached regarding its safety. We have systematically synthesized GO nanosheets with high control and homogeneity of size and minimal trace metal and no endotoxin contaminations that do not exert the overt toxicity reported for many commercial sources of other graphene material types (such as graphene nanoplatelets). These highly purified GO materials have been thoroughly investigated using in vitro and in vivo models by different laboratories during the past decade. A recent repeated and long-term pulmonary exposure study of these GO nanosheets in mice found that large (micrometre range) lateral size and high doses induced pulmonary inflammation (although substantially less than long, rigid carbon nanotubes) and more persistent granulomas. Smaller (nanometre range) GO nanosheet exposures have demonstrated a transient inflammatory response that resolved rapidly post-exposure.
Parallels can be drawn between manufactured nanomaterials and the small particulate matter in air pollution. Particulate matter exposure has been linked to adverse health effects in almost every organ of the body, although the respiratory and cardiovascular effects drive the substantial morbidity and mortality associated with particulate matter. Ultrafine (nano-sized) particulate matter is likely to contribute to these effects, given the high deposition in the alveoli of the lungs, the high reactive surface area for a given mass and the penetration to systemic organs. While different classes of manufactured nanomaterial have distinct properties, there are commonalities with ultrafine particulate matter with respect to some physiochemical features and the pathways by which they induce toxicological effects, such as inflammation and oxidative stress. Although there is an expanse of large-cohort epidemiological data linking particulate matter in air pollution with adverse effects, human data on the biological actions of manufactured nanomaterials are confined to cultures of human cell lines and biomonitoring in occupational settings or isolated accidental exposures in small groups of individuals. Inhalation is the primary route of unintended pulmonary exposure to manufactured nanomaterials, but it also represents a promising route of administration for nanomedicines used for diagnosis and drug delivery for respiratory conditions. Therefore, human data are urgently needed to assess risk assessment and realize the true potential of these materials.
Controlled human exposure studies offer several advantages for assessing the acute biological effects of xenobiotics. Unlike epidemiological studies, substances can be tested in isolation at defined doses. Real-world confounders, such as other environmental stressors (for example noise, stress, heat, exercise or medication) can be minimized or standardized across participants and study visits. The design can be tailored to include relevant control exposures, with repeated measure designs allowing each participant to be their own control. The use of a controlled exposure environment at clinics or laboratory facilities can broaden the range of endpoints to include a greater range of subclinical and mechanistic endpoints and has been used successfully to determine the respiratory and cardiovascular effects of combustion-derived nanoparticles such as those in diesel exhaust emissions. However, only a handful of studies have tested the actions of manufactured nanomaterials, and no studies have been performed with non-spherical materials such as graphene.
In this study we aimed to understand the potential for GO to have detrimental health effects, principally from the viewpoint of unintended exposure (for example occupationally or from public exposure with increasing use of nanomaterials in real-world applications) but also from the perspective of the development of safe forms of GO for intended human exposure by inhalation (for example for diagnostic imaging of the lung or drug delivery to or via the lung). Using a randomized controlled double-blind crossover design, we investigated the cardiorespiratory effects of acute inhalation of GO nanosheets in human volunteers. We hypothesized that inhalation of our high-purity, thin GO would have only modest effects on cardiorespiratory function and blood markers of inflammation and coagulability, the magnitude of which would be lower the smaller the lateral dimensions of the nanosheets. The findings of this controlled human inhalation exposure to GO in human volunteers lay the foundations for future investigations to establish which properties of graphene materials determine their biological actions to provide a formal risk assessment and allow safe-and-sustainable-by-design development for various applications.
Synthesis and Characterization of GO Nanosheets
Thin, highly purified metal-free and endotoxin-free GO materials were synthesized using a modified Hummers’ method and comprehensively characterized (Fig. 1, Extended Data Table 1 and Extended Data Fig. 1). GO nanosheets were of tightly defined size, with no metallic or other elemental contamination, residues or indicators of bacterial contamination. Two lateral dimensions (maintaining all other physicochemical characteristics almost identical) were selected for the study: small GO (s-GO) and ultrasmall GO (us-GO). Both types of nanosheet have demonstrated no acute or longitudinal adverse effects in our previous pre-clinical (rodent) studies, contrary to ‘large’ GO sheets that were thus excluded from this work as a safety precaution.
Fig. 1: GO Nanosheet Size Distributions
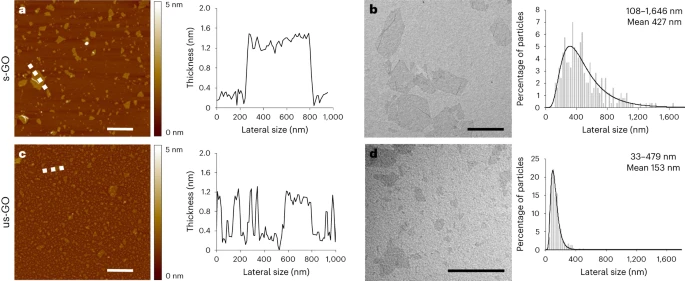
a–d, Morphological characterizations of s-GO (a,b) and us-GO (c,d). The images (left) and spectra (right) are representative of the technical replicates performed. Atomic force microscopy height images (left; a and c) with the corresponding cross-section analysis along the indicated dashed white lines (right; a and c). The colour bars indicate the height intensity range from 0 to 5 nm. Transmission electron microscopy micrographs (left; b and d) with the size distribution analyses using a Gaussian single peak fitting depicted with solid lines (right; b and d) are shown. Scale bars are 1 µm. The lateral size range and means given in b and d are an average of 242 and 224 individual GO sheets, respectively (see a summary table in Extended Data Table 1).
GO nanosheets were aerosolized for exposure of volunteers through inhalation via a face mask with a target mass concentration of 200 μg m−3. The actual concentrations of s-GO and us-GO were 214 ± 23 μg m−3 and 224 ± 17 μg m−3, respectively (Extended Data Table 2) and were maintained at a constant level throughout the 2 h exposure (Supplementary Fig. 1a). As anticipated, the us-GO had a greater particle number than the s-GO for the same mass. The GO agglomerated to airborne sizes with a median value of 80–90 nm and a high size distribution homogeneity (Supplementary Fig. 1b). The levels of GO used here (200 μg m−3) were substantially higher than concentrations of graphene materials found in many workplaces that handle/process these materials (0.4–50 μg m−3) and are relevant to proposed occupational guidance for graphene nanoplatelets (212 μg m−3).
